Descripción
San Francisco, CA Quality by Design
Agenda
NOTE: One-day course participants will obtain a conceptual understanding, while five-day course participants will obtain more detailed and advanced understanding through a combination of hands-on exercises and analysis of various case studies.
- Background and drivers for Pharma QbD
- QbD guidance review (ICH Q8/Q9/Q10/Q11)
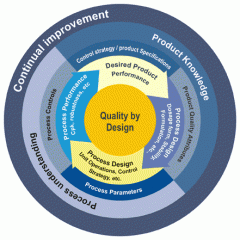
- QbD development process and flow/logic
- Introduction and overview of QbD tools
- Risk management
- Design of Experiments
- Design Space
- Control Strategies
- Current industry and regulatory landscape, and application challenges for QbD
- Focused hands-on DoE training and practice
- Full vs fractional factorials
- Optimization and response surfaces
- Transfer functions
- QTPP and CQAs/CMAs/CPPs
- Risk management tools and practices
- Design space: development, verification and scale-up
- Control strategies, process robustness and proactive management of process variation
Objectives/Learning Outcomes
- Understanding the origins and need for QbD for pharmaceutical products, and the objectives for current QbD practices
- Understanding the overall logic and flow of the QbD development process, including the use and application of the various tools applied:
- Risk-based quality management
- Development and use of Quality Target Product Profiles (QTPPs), and definition and verification of Critical Quality Attributes (CQAs), Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs)
- Facets of statistical design of experiments (DOEs), e.g., screening designs, response surfaces and optimization, transfer functions
- Design Space and its application: process control, lifecycle management, understanding design space limits/“edges”
- Development and optimization of process control strategies, including real-time release testing.
- The ability for attendees to apply these QbD tools and principles to their own problems, facilitated through round-table discussion of challenges faced by attendees